Suntan Construction details of a wet aluminum electrolytic capacitor - Production process
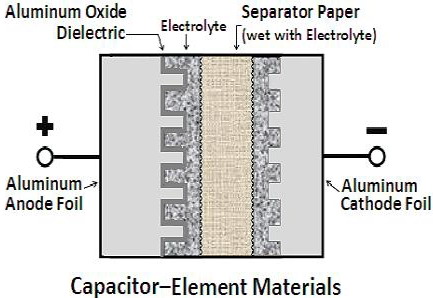
Aluminum electrolytic capacitors are comprised of anode and cathode plates separated by an absorbent spacer. As shown in Figure below, metal tabs are attached to the anode and cathode plates, and the assembly is wound into a cylindrical section. The tabs are welded to aluminum terminals installed in a header (top). The section-header assembly is immersed in a bath of hot capacitor electrolyte (significantly different from the formation process electrolyte). In what is called the impregnation process, a vacuum is applied to the electrolyte and sections, causing electrolyte to be drawn into the sections, thoroughly wetting the sections. The sections are placed in aluminum cans, and the headers are sealed to the cans. The capacitor units are slowly brought up to maximum rated voltage at maximum rated temperature during the aging process. The aging process grows oxide on areas on the anode foil which have an insufficient oxide barrier, such as slit edges and places which have been cracked during the winding operation. Inspections and tests occur at several stages of the production process.